Dithiaethynporphyrin
This molecule was obtained by A. Berlicka on a route of [3+1] condensation.
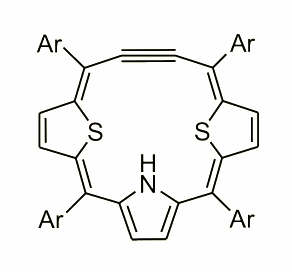
Incorporation of an acetylene fragment into a porphyrin frame results in formation of 18p electron macrocyclic delocalization pathway which can be described as a combination of acetylene and cumulene character of the acetylene fragment. The 13C NMR chemical shifts of dithiaethynporphyrin point out that both canonical structure contribute to the overall electronic structure and well correspond to previously obtained acetylene-cumulene porphyrinoids.
| Further exploration of the macrocycle coordination abilities resulted in the synthesis and charaterisation of the Fe(II) double decker complex. The spectroscopic properties together with the dynamic behaviour of this paramagnetic molecule were explored by several 1H NMR experiments. The exploration has shown a free rotation of two organic subunits around the axis passed through the metal centre. | 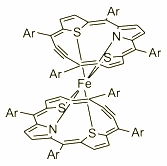 |
Further reading
Berlicka, A.; Latos-Grażyński, L.; Lis, T. Dithiaethynporphyrin – an Atypical [18]Triphyrin(4.1.1) Frame for Contracted Porphyrins Angew. Chem. Int. Ed. 2005, 44, 5288.
Berlicka, A.; Latos-Grażyński, L. Intramolecular Rotation of Iron(II) Dithiaethyneporphyrin Double-Decker Complex: 1H NMR Studies Inorg. Chem. 2009, 48, 7922.
(See the complete publication list of our group.)


