

5,10,15,20-Tetraaryl-22-hetero-1,5-naphthiporphyrins, which contain a 1,5-naphthylene moiety instead of one pyrrole embedded in the macrocyclic framework of heteroporphyrins, were obtained by the [3 + 1] approach by B. Szyszko.
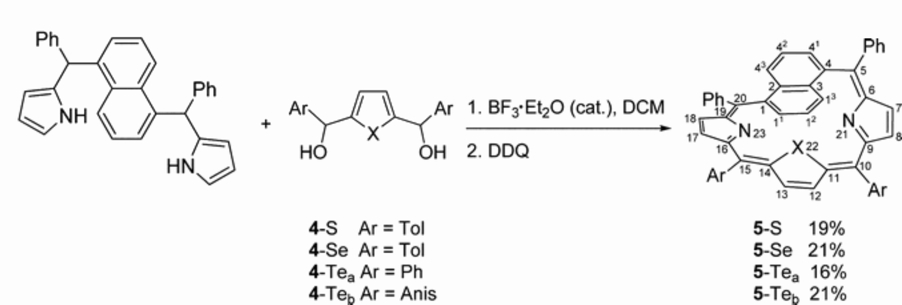
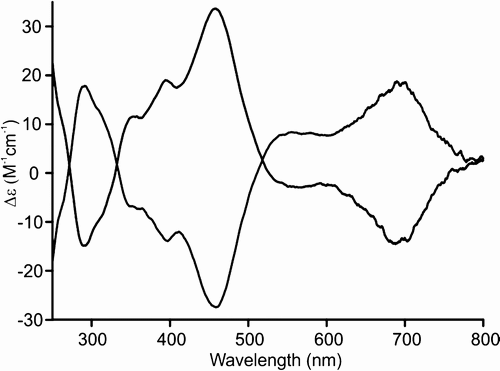
The steric constraints, imposed by the substitution mode of the 1,5-naphthylene building block, resulted in the specific helical conformation of 22-hetero-1,5-naphthiporphyrins. The spectroscopic and structural properties of these aceneporphyrinoids indicate a lack of macrocycle aromaticity.
Further reading
Szyszko, B.; Pacholska-Dudziak, E.; Latos-Grażyński, L. Incorporation of 1,5-Naphthalene Subunit into Heteroporphyrin Structure - toward Helical Aceneporphyrinoids. J. Org. Chem. 2013, 78, 5090