Expanded Porphyrins
Tetraarylsapphyrin was discovered by K. Rachlewicz as a byproduct of the Lindsey-type tetraarylporphyrin synthesis.
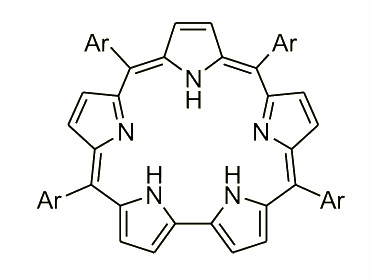
The molecule contains a bipyrrole unit replacing one of the pyrrole rings and is aromatic. An unusual conformational feature of tetraarylsapphyrin was the inversion of the "central" ring. In the free base this ring is "inverted", i.e. directs its nitrogen atom outwards. In the dication, however, the ring flips back into the usual position with the N atom inside the macrocycle.
The molecule presented above is an example of expanded porphyrin, where the number of subunits has increased. During the exploration, and a careful analysis of the condensation mixture several examples of such molecules have been reported. An extraordinary molecule was observed by N. Sprutta and named a giant porphyrin. This heteroporphyrinoid was synthesized in an acid-catalyzed condensation of pyrrole and 2,5-bis(p-tolylhydroxymethyl)thiophene.
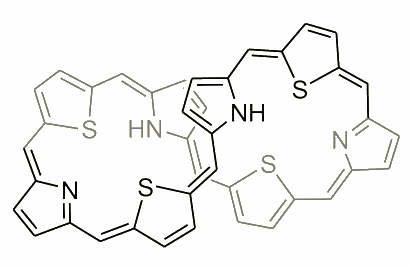
The new compound is denoted as S4OP, which stands for 5,10,15,20,25,30,35,40-octa(p-tolyl)-41,43,45,47-tetrathia-[36]octaphyrin(1.1.1.1.1.1.1.1). It is reversibly reduced to S4OPH2, i.e. 5,10,15,20,25,30,35,40-octa(p-tolyl)-dihydro-41,43,45,47-tetrathia[38]octaphyrin(1.1.1.1.1.1.1.1). Both S4OP and S4OPH2 adopt figure-eight conformations in solution. In each case the molecule is very flexible, which gives rise to dynamic broadening of the NMR spectra. In S4OP the positions at the figure-eight crossing are occupied by thiophene rings, whereas in the reduced form they are occupied by pyrroles. S4OP exhibits residual paratropic shifts in 1H NMR corresponding to the "antiaromatic" conjugation pathway (36pi electrons). The 38-electron pathway of S4OPH2 results in residual diatropicity of the molecule.
Kontekstowa uwaga dla polskich czytelników
Tetratia[36]oktafiryna(1.1.1.1.1.1.1.1) znana jest w naszym slangu laboratoryjnym pod nazwą Ludwik, którą zawdzięcza charakterystycznemu kolorowi przypominającemu legendarny płyn do mycia naczyń.
Further reading
Chmielewski, P. J.; Latos-Grażyński, L.; Rachlewicz, K. 5,10,15,20-Tetraphenylsapphyrin - Identification of a Pentapyrrolic Expanded Porphyrin in the Rothemund Synthesis. Chem.Eur.J. 1995, 1, 68.
Sprutta, N.; Latos-Grażyński, L. Figure-Eight Tetrathiaoctaphyrin and Dihydrotetrathiaoctaphyrin. Chem.Eur.J. 2001, 7, 5099.
(See the complete publication list of our group.)


