Silaphlorin
Work of J. Skonieczny on core-modified porphyrins led to generation of silicon-modified core of 21-silaphlorin. Attempts to trap 21-silaporphyrin resulted in the serendipitous discovery of a unique transformation of 21-silaphlorin into a non-aromatic isomer of 2,3-diphenyl-5,10,15,21-tetra(p-tolyl)-carbacorrole (iso-carbacorrole).
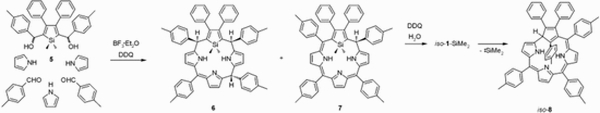
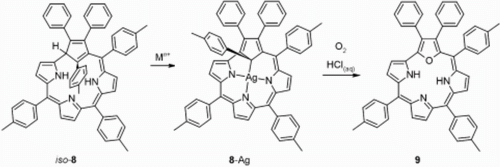
Insertion of silver or copper ions into iso-carbacorrole gave two structurally related organometallic complexes of “true” carbacorrole in which the metal(iii) ions are bound by three pyrrolic nitrogen atoms and a tetrahedrally hybridised C21 atom of the cyclopentadiene moiety. In the presence of oxygen, the silver(iii) carbacorrole undergoes internal oxidation to 21-oxacorrole.
Further reading
Skonieczny, J.; Latos-Grażyński, L.; Szterenberg, L. Reactivity of Silole within a Core-Modified Porphyrin Environment: Synthesis of 21-Silaphlorin and its Conversion to Carbacorrole. Chem. Eur. J. 2008, 14, 4861.


