m-Benziporphyrins
The coordination chemistry of meta-benziporphyrin and its derivatives with rhodium(III), ruthenium(II) and gold(III) has been studied by K. Hurej.
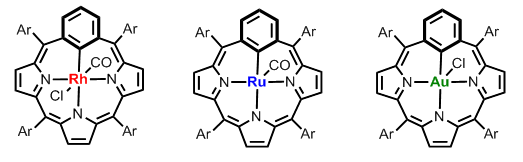
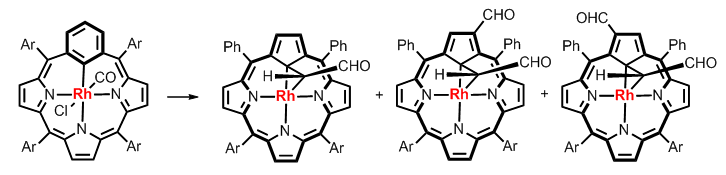
In course of exploration, we have observed an unprecedented reactivity of rhodium(III) m-benziporphyrin resulting in a transformation of the built-in m-phenylene with an extrusion of a perimeter carbon what eventually gave rhodium(III) 21-carbaporphyrin, which stabilizes rhodacyclopropane motif substituted by a formyl unit.
The analysis of mentioned contraction allowed determining the structural frames of macrocycle critical for controlled and efficient transformation of meta-phenylene. Based on those predictions two new types of ligands were obtained – 22-methyl- and 22-ethyl-meta-benziporphyrin. Insertion of rhodium to both ligands leads to aromatic complexes obtained quantitatively and showing an involvement of methyl or ethyl to coordination of transition metal as bridging donors.
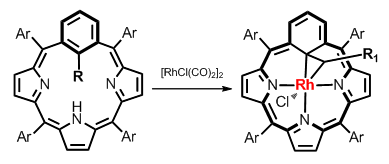
The reactivity of gold(III) 22-methyl-meta-benziporphyrin have been explored showing an effective (close to quantitative) transformation of m-phenylene ring embedded in the equatorial ligand to cyclopentadiene substituted with formyl functionality.
Further reading
Hurej, K.; Pawlicki, M.; Szterenberg, L.; Latos-Grażyński L. A Rhodium Mediated Contraction of Benzene to Cyclopentadiene. Transformations of Rhodium(III) m-Benziporphyrin. Angew. Chem. Int. Ed. 2016, 55, 1427
Hurej, K.; Pawlicki, M.; Latos-Grażyński, L. Gold(III) triggered transformations of 22-methyl-m-benziporphyrin involving an effective contraction of benzene to cyclopentadiene. Chem. Eur. J. 2017, 23, 2059.


