Ferrocenoporphyrin
Ferrocenothiaporphyrin was obtained by I. Simkowa in a [3+1] macrocyclization reaction that involved 1,1’-bis[phenyl(2-pyrrolyl)methyl]- ferrocene and 2,5-substituted thiophene, which were condensed under Lindsey-type conditions followed by a DDQ oxidation.
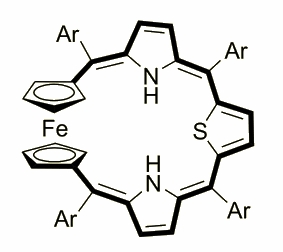
Depending on the oxidation state the molecule presents features characteristic for aromatic or antiaromatic chromophore. The ferrocene molecule built-in the macrocycle frame shows a hitherto unknown facet of porphyrinoind aromaticity - the possibility of including a d-electron in a pi-electron conjugation pathway.
Further reading
Simkowa, I.; Latos-Grażyński, L.; Stępień, M. π Conjugation Transmitted across a d-Electron Metallocene in Ferrocenothiaporphyrin Macrocycles Angew. Chem. Int. Ed. 2010, 49, 7665.
(See the complete publication list of our group.)


