Oxatriphyrins(2.1.1)
M. Pawlicki with K. Hurej and M. Garbicz obtained a set of oxatriphyrins(2.1.1).
Thiophene-fused triphyrins(2.1.1) were synthesized by applying an innovative approach. Spectroscopic techniques show that macrocycles are aromatic and quantitatively convert into anti-aromatic structures after reduction. The reduced forms were stabilized through boron(III) coordination, thereby allowing the observation of anti-aromatic 16 π delocalization within a contracted porphyrin.
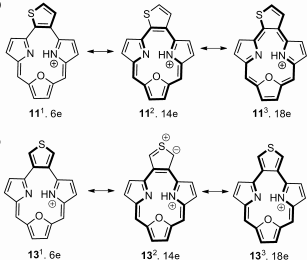
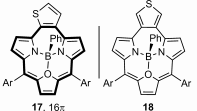
When ortho-substituted variant is introduced into a triphyrin(2.1.1) skeleton an aromatic molecule is obtained and the carbocyclic ring participates in the conjugation of the macrocycle. The two-electron reduction and introduction of boron(III) changes the aromatic character and results in an anti-aromatic structure.
Further reading
Pawlicki, M.; Hurej, K.; Szterenberg, L.; Latos-Grażyński, L. Synthesis and Switching the Aromatic Character of Oxatriphyrins(2.1.1). Angew. Chem. Int. Ed. 2014 53, 2992.
Pawlicki, M.; Garbicz, M.; Szterenberg, L.; Latos-Grażyński, L. Oxatriphyrin(2.1.1) Incorporating an ortho Phenylene Motif. Angew. Chem. Int. Ed. 2015, 54, 1906.


