Properties
Porphyrins are strongly colored compounds. The variety of available colors is apparently unlimitedalthough there is an observable inclination towards red and purple. The color is a consequence of the complicated electronic spectra of porphyrins, which contain intense absorptions in the visible region (called Q bands). Even more intense (ten times and more) is the Soret band found in the near UV, so named after its discoverer (Soret, J. L. Compt. Rend. 1883, 97, 1267).
Another conspicuous characteristic of porphyrins, directly associated with their optical properties,is the aromaticity of the macrocycle. It is most evidently manifested in the 1H NMR spectra by the downfield shifts of peripheral protons and the strongly upfield shifts of the inner NH's(usually negative on the d scale).
Porphyrin aromaticity is most frequently described in terms of the [18]annulene model, proposed by E. Vogel. According to that model, a delocalization pathway is distinguished in the macrocycle, as shown in Figure 4, which is aromatic in the traditional Hückel sense. The porphyrin is thus viewed as a bridged diaza[18]annulene.
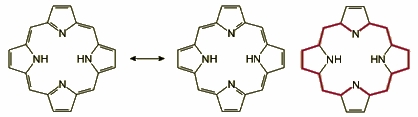
Fig. 4. Delocalization pathway in porphyrin according to Vogel's [18]annulene model.
Porphyrins are weak bases and can be protonated to form dications. In the unprotonated porphyrin (called free base) the two inner protons are mobile and jump freely among the four nitrogens. The trans (21,23-H) tautomer, shown in all schemes, is energetically preferred to the cis form (21,22-H).
Porphyrins form a great number of complexes with metal ions and some nonmetals (Figure 5).The coordinating environment provided by porphyrins is very flexible and can be fine-tuned to particular oxidation and spin states by varying peripheral substitution and axial ligands. This tunability was probably Nature's reason to choose porphyrin as its "workhorse macrocycle."
Of the naturally ocurring metalloporphyrins the iron complexes, called hemes, are by far the most important. They make the reactive centers of numerous heme proteins, responsible for oxygen transport and storage (hemo- and myoglobin), electron transfer (cytochromes), and oxidation of organic substrates (oxygenases of the P450 family).
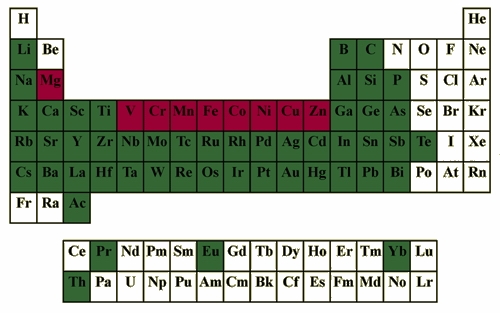
Fig. 5. Elements known to form complexes with porphyrins (green). Metals found in Nature are shown in red.


