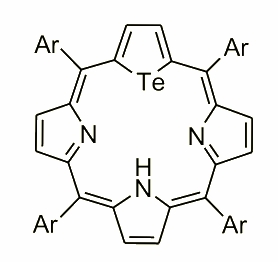
Telluraporphyrins
Modification of the coordination core by replacing one of the inner nitrogens covers a wide scope of heteroatoms. From one point of view introducing the oxygen atom with formation of 21-oxaporphyrin creates a fantastic environment for exploring the coordination properties toward lower, atypical oxidation states of transition metals, i.e. nickel(I) or iron(I). On the opposite side of this special character of 21-heteroporphyrin stays a tellurium analogue with the heteroatom that’s atomic radius shrinks the cavity size and blocks the abilities to coordinate any transition metal. Nevertheless employment of this moiety did not affect the spectroscopic properties observed for the final macrocycle. It possesses an aromatic character easily noticeable with a little help from the spectroscopic analysis.
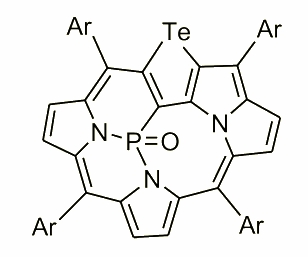
This molecule obtained by E. Dudziak has shown an extraordinary behaviour while reacted with PCl3. In this case the tellurophene has flipped into the coordination cavity and fused with one of the nitrogens creating a core able to bind phosphorous.
This dynamic process with a tellurium pointed outwards and here forced by the central atom was for the first time observed for ditelluraporphyrin obtained on a way of a ‘typical’ core-modification approach. Acid catalysed condensation with 2,5-substituted tellurophene and pyrrole gave 21,23-ditelluraporphyrin.
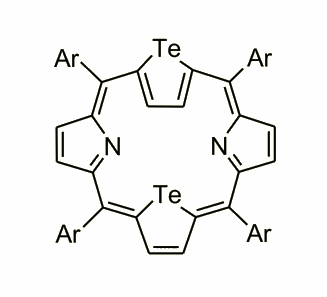
The size of the tellurium atoms is instrumental in the remarkable distortion of macrocycle, which was proved by X-ray analysis. One of the tellurophene rings is inverted similarly as the unique pyrrole in tetraarylsapphyrin. The macrocycle of ditelluraporphyrin is flexible and the two tellurophenes invert alternately switching the molecule between two equivalent conformations.
Further reading
Latos-Grażyński , L.; Pacholska, E.; Chmielewski, P. J.; Olmstead, M. M.; Balch, A. L. Alteration of the Reactivity of Tellurophene Within a Core-Modified Porphyrin Environment. Synthesis and Oxidation of 21-Tellurapophyrin Angew.Chem., Int.Ed.Engl. 1995, 107, 2252.
Pacholska, E.; Latos-Grażyński , L.; Ciunik, Z. An Unorthodox Conformation of [18]Porphyrin(1.1.1.1) Heteroanalogue- 21,23-Ditelluraporphyrin With Flipped Tellurophene Ring Angew.Chem., Int.Ed.Engl. 2001, 40, 4466.
Pacholska-Dudziak, E.; Ulatowski, F.; Ciunik, Z.; Latos-Grażyński, L. N-Fusion Approach in Construction of Contracted Carbaporphyrinoids: Formation of N-Fused Telluraporphyrin Chem. Eur. J. 2009, 15, 10924
(See the complete publication list of our group.)


