Vacataporphyrins
In a special approach to porphyrin chemistry, these outstanding macrocycles can be treated as bridged annulenes, where nitrogens play role of special linkers. From this point of view another possibility of modification comes from not a simple replacing the core nitrogen by another heteroatom, but by removing the bridge (NH group) that leads to formation of the annulene-porphyrin hybrid and brings a new way of creation non-trivial ligands. The first example of macrocycles like that was prepared by E. Dudziak on a way of an acid catalyzed removing of a tellurium from 21-telluraporphyrin.
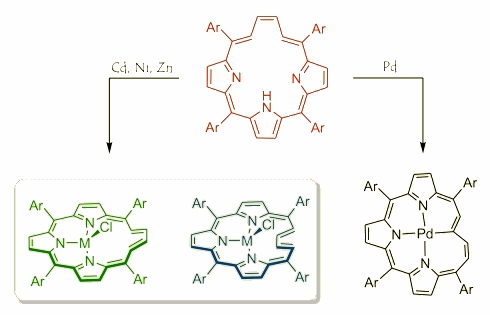
This molecule keeps the spectroscopic properties typical for porphyrin-like macrocycles and possesses a diatropic current in the 1H NMR together with absorption properties characteristic for aromatic porphyrinoids. Compounds like that create an extraordinary opportunity to explore the coordination properties of macrocycles with a flexible fragment linking a tripyrrolic brace. A reaction between vacataporphyrin and metal(II) salts (zinc(II), cadmium(II) and nickel(II)) gave in every case a mixture of two compounds where the tetracarbon bridge occupies two opposite geometries – outwards and inwards while taking the metal centre as a differencing element. While the palladium(II) was introduced to the reaction the formation of the third coordination mode was observed with a direct metal-carbon bond. In addition flexibility of the butadiene allowed observing a Mobius topology by twisting a tetracarbon bridge forced by an interaction with a metal centre.
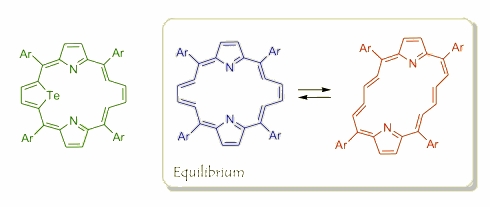
Further exploration of this reactivity gave additional members of the vacata- family. Similar conditions applied to ditelluraporphyrin gave two new macrocycles, where just one (green) or both (blue) telluriums have been removed. Also in these cases the spectroscopic properties locate both macrocycles in a row along with other porphyrin-like compounds. Nevertheless the flexibility of both butadiene bridges brings a dynamic to the whole system that allowed observing a cis-trans isomerisation documented with help of a temperature dependent NMR experiments.
Further reading
Pacholska, E.; Latos-Grażyński, L.; Ciunik, Z. A Direct Link Between Annulene and Porphyrin Chemistry 21-Vacataporphyrin Chem.Eur.J. 2002, 8, 5403.
Dudziak, E.; Skonieczny, J.; Pawlicki, M.; Latos-Grażyński, L.; Szterenberg, L. Cadmium(II), Nickel(II) and Zinc(II) Complexes of Vacataporphyrin - A Variable Annulene Conformation Inside a Standard Porphyrin Frame, Inorg. Chem. 2005, 44, 8794.
Pacholska-Dudziak, E.; Skonieczny, J.; Pawlicki, M.; Szterenberg, L.; Ciunik, Z.; Latos-Grażyński, L. Palladium Vacataporphyrin Reveals Conformational Rearrangements Involving Hückel and Möbius Macrocyclic Topologies J. Am. Chem. Soc. 2008, 130, 6182.
Pacholska-Dudziak, E.; Szterenberg, L.; Latos-Grażyński, L. A Flexible Porphyrin-Annulene Hybrid - a Non-Porphyrin Conformation Caught for meso-Tetraaryldivacataporphyrin Chem. Eur. J. 2011, 17, 3500.
(See the complete publication list of our group.)


